
NNIT teams up with Danish e-sports organization Astralis to explore learning through gamification
News • Apr 25, 2024 07:45 GMT
Together with Astralis, we are currently exploring insights from game studies as an alternative to traditional e-learning for public IT systems, considering both the case handler and citizen perspectives.
Today, most interfaces are digital, and as employees and citizens we access and work in fairly complex public IT systems as we go through life. Learning how to process applications, tax filings etc. correctly is not always easy and almost certainly tedious; and our working hypothesis is that it is particularly difficult to motivate the under 30s to access and interact with public IT systems.
But what if onboarding and learning was made fun because it was built like a game?
Recently, as a first step in our collaboration, we attended the conference OffDig with Astralis to initiate discussions with our public customers about their challenges in this area and to propose that insights from game studies might offer viable solutions.
“We continue to have great dialogues with our customers and Astralis based on pain points in traditional learning approaches, which leads us to believe that our collaboration with Astralis will bring new ways of using and learning about IT systems in the Danish public sector,” says Business Development Lead Jannic Stolzenbach Jensen, NNIT, who was part of this year’s OffDig delegation.
As a next step, we will begin to mature our ideas, which should ultimately produce a number of concrete offerings to the public sector in terms of gaming modules that may be used to train case handlers as well as onboard citizens to public IT systems such as Digital Post, the Danish students' Grants and Loans Scheme, Borger.dk etc.
We look forward to a fruitful collaboration with Astralis as well as our customers to make public IT systems more accessible and fun.

NNIT Releases the Valiance TRUseries 5.0 – Revolutionizing Data Migration with AI and Enhanced Performance Features
Pressrelease • Apr 18, 2024 15:30 GMT
NNIT’s Migration Powerhouse, renowned for its expertise in critical data migrations where compliance and accuracy are paramount, is proud to announce the launch of the latest iteration of its flagship software, Valiance TRUseries 5.0. This release marks a significant advancement in data migration technology, introducing innovative features that promise to redefine the standards for efficiency, data integrity, and optimization in the data migration process.
Refined through well over 800 migrations for 190 global customers in the life sciences and bio-pharma sectors, Valiance TRUseries 5.0 is meticulously designed to meet the stringent requirements of regulated industries, offering unparalleled technology, compliance, and 100 percent data verification.
Key Features and Benefits
Migration Simulation and Data Discovery: The inclusion of migration simulation and the advanced Data Discovery functionality in TRUseries 5.0 allows for comprehensive pre-migration testing and data quality assessment. This feature not only saves valuable time and resources but also guarantees a more refined and accurate data migration process and its outcome.
AI-Assisted Document Classification and Enrichment: By harnessing the power of AI and ML (Machine Learning), TRUseries 5.0 addresses the critical challenge of preparing incomplete data for migration. This AI-driven approach significantly reduces manual intervention, ensuring data is migration-ready and meets the stringent validation rules of the target system.
Mapping Variables for Multi-Stage Transformation: With the introduction of Mapping Variables, TRUseries 5.0 enhances the flexibility and precision of data migration. This feature allows for the creation and utilization of new identifiers in data transformation, paving the way for a more nuanced and effective migration strategy.
"We're incredibly excited about the launch of TRUseries 5.0. This version represents the culmination of a multi-million-dollar investment to ensure our solution adheres to strict quality standards and our deep commitment to innovation, tailored specifically for the highly regulated environments of life sciences. Our goal has always been to provide our customers with not just a tool, but a comprehensive solution that addresses the complexities of data migration. With TRUseries 5.0, we're setting a new benchmark for what technology can achieve in terms of speed, accuracy, and compliance," says Nagesh Sarma, Associate Vice President, at NNIT.
This latest version of Valiance TRUseries is a testament to NNIT's ongoing pursuit of excellence and its commitment to supporting customers through their digital transformation journeys with the most advanced, secure, and compliant data migration solutions available.

NNIT reinforces AI focus and investments by anchoring regional business units in new global structure led by Director Sam Laermans
Pressrelease • Apr 09, 2024 08:30 GMT
A new global structure dedicated to AI and led by Director Sam Laermans has been created to ensure unity, best practice, and maximum utilization of expertise and resources across our four regional AI business units.
The new, global structure will be responsible for NNIT’s overall AI strategy, partnerships, and models as well as building and aligning AI solutions and services offered and tailored to our markets: Private & Public in Region Denmark, and Life Sciences in USA, Europe, and Asia.
By the end of 2024, the global structure will comprise of at least 15 dedicated employees across our AI units in Denmark, USA, Europe, and Asia.
“AI is neither a new technology or new to NNIT, but with the increased interest and appetite for using this technology to aid and support human resources in achieving further optimizations and efficiencies, we wish to reinforce our efforts and investments in a global structure to ensure best practice and the creation of a veritable AI powerhouse. This will position us well to address specific opportunities in our markets and add further value to our customers,” says CEO Pär Fors.
During the coming months, the global structure will come into its own with NNIT’s overall AI strategy and regional business plans ready for implementation in June. The regional AI unit leads are Maria Baun Marcker for Denmark, Dane Stout and Nagesh Sarma for USA, Sam Laermans for Europe (doubling as global lead), and John Yin Zhao for Asia.
“After an explorative 2023, we are seeing a new level of seriousness around the AI technology with high impact use cases in advanced data management, analytics, and AI powered automations. This opportunity is matched with new regulatory requirements such as the EU AI Act and new validation strategies, which challenges the IT and business boundaries further than ever before. With a strong global structure in place, I look forward to guiding our customers through these challenges into a new era of AI and digital transformation,” says Director Sam Laermans.

The NNIT China head office relocates to support our development in the region
News • Mar 25, 2024 09:58 GMT
Our China head office has relocated from Tianjin Jinwan Mansion to Tianjin Luneng International Center to provide employees with a more convenient and flexible office environment as well as better surroundings for the collaboration with our customers and partners.
Region Asia is an important part of the overall NNIT 2026 strategy where we are focusing our business goals at the international Life Sciences market and the public section in Denmark.
For Region Asia we aspire to get more local market share, build domain and tech competencies in a close collaboration between the Tianjin and Singapore office, as well as being closer to our customers to support our development in the Chinese market.
As Head of Region Asia, Jason Xing says:
“This year is the 18th year of NNIT coming to China. We have already established our brand awareness and have 300 talented employees across 3 different offices. Last year, we had a relocating of our Shanghai office. And now, we have the relocating of the Tianjin Office. With the new locations our employees get a more inviting and open working environment and equally important, our customers will also get a better impression about us as a global IT consultancy working hard every day to be the best possible digitalization partner.”
At our new location, we can also look forward to better possibilities for attracting new talent.
HR director of Region Asia, Nancy Liu explains:
“The new location is nearby several top universities in China located in Tianjin. It provides more possibility for attracting outstanding talents to join us through our Student Program, which also gives a competitive position of retaining talent and supporting career development in NNIT. All this will make us become an employer of choice.”
NNIT China contact details:
34th Floor, Luneng International Center
East of Water Park Road,
Nankai District, Tianjin
China
Tel. +86 (22) 58856666

The NNIT China head office has been relocated to support our development in the region
News • Mar 25, 2024 09:58 GMT
Our China head office has relocated moving from Tianjin Jinwan Mansion to Tianjin Luneng International Center to provide employees with a more convenient and flexible office environment as well as better surroundings for the collaboration with our customers and partners.
Region Asia is an important part of the overall NNIT 2026 strategy where we are focusing our business goals at the international Life Sciences market and the public section in Denmark.
For Region Asia we aspire to get more local market share, build domain and tech competencies in a close collaboration between the Tianjin and Singapore office, as well as being closer to our customers to support our development in the Chinese market.
As Head of Region Asia, Jason Xing says:
“This year is the 18th year of NNIT coming to China. We have already established our brand awareness and have 300 talented employees across 3 different offices. Last year, we had a relocating of our Shanghai office. And now, we have the relocating of the Tianjin Office. With the new locations our employees get a more inviting and open working environment and equally important, our customers will also get a better impression about us as a global IT consultancy working hard every day to be the best possible digitalization partner.”
At our new location, we can also look forward to better possibilities for attracting new talent.
HR director of Region Asia, Nancy Liu explains:
“The new location is nearby several top universities in China located in Tianjin. It provides more possibility for attracting outstanding talents to join us through our Student Program, which also gives a competitive position of retaining talent and supporting career development in NNIT. All this will make us become an employer of choice.”
NNIT China contact details:
34th Floor, Luneng International Center
East of Water Park Road,
Nankai District, Tianjin
China
Tel. +86 (22) 58856666

NNIT’s Marina Prodan selected as Talent 100
News • Mar 22, 2024 07:30 GMT
More than 400 talented and ambitious young people were nominated for this year's Talent 100 published by the Danish broadsheet newspaper Berlingske. Only 100 talents were ultimately selected for this year’s list, and Marina Prodan is one of them.
Marina Prodan’s story is quite extraordinary. At age 19, in 2010, she arrived alone in Denmark from Romania to study business administration at Copenhagen Business School. Determined to put down roots and launch an accelerated business career, she quickly set out to learn Danish, which is not an easy undertaking.
While learning Danish and studying, she worked odd jobs to support herself. In record-time, her Danish was good enough for her to land a steady job as a cashier in a supermarket. Next, she landed a job as a student assistant in a consulting company – and after five years, she graduated with an MSc. in Economics and Business Administration.
An accelerated career with NNIT
Having finished her studies, Marina Prodan immediately found full-time employment with NNIT where she was recognized as an extremely likable colleague and valued customer liaison. She quickly climbed the ranks, and today, she is the director of NNIT’s globally leading Regulatory Affairs consulting unit in Europe.
“Marina always rises to the occasion and is equally valued by colleagues and customers for her ability to listen and deliver results. She is an obvious talent and so deserving of the selection as one of the Talent 100 for 2024, says Vice President Niels Buch Leander and continues:
“She is a modern leader with the ability to successfully liaise with colleagues, customers, and partners to achieve common goals and success. She has driven growth within her areas of responsibility, not least doubling the revenue with one central life sciences customer. Marina’s determination, ambition and leadership style deserves recognition and praise; we think that she is one of Denmark’s greatest talents in the consulting arena, and we know that she is a role model, particularly for our female consultants.”
To infinity and beyond?
Marina Prodan is honored to have been selected and comments: “I feel beyond proud and happy. To me, being on this year’s Talent 100 list is the culmination of my 14-year journey in Denmark so far, from making ends meet while in school and learning Danish to really making my mark at NNIT. I am immensely grateful that almost eight years ago, a wonderful hiring manager at NNIT took a chance on me and helped pave the way for where I am today, even though back then my business Danish wasn’t perfect, and I still had limited work experience.”
So, what’s next for Marina? We asked her if there is a career plan v2 in the making?
“Of course there is, but I will wait a bit with spilling the beans. What I can say is that, currently, the life sciences industry is evolving at a rapid pace, and at NNIT, we are spending quite some time on advising our customers on the next big things in Regulatory Affairs, including topics such as data management, IDMP and generative AI. So, for now, my focus is on accelerating the journey that my department is on and expanding our overall European footprint. From there on, the sky is the limit.”
Marina Prodan’s accelerated career path at NNIT:
- 2016-2019: Business Consultant
- 2019-2021: Advanced Business Consultant
- 2021: Senior Business Consultant
- 2021: Promotion to Line manager
- 2023: Promotion to Manager of the European life sciences consulting unit (managing 15 consultants in Germany, UK, Switzerland, Denmark, and Poland).
- 2023: Promotion to Director responsible for NNIT’s Regulatory Affairs consulting unit in Europe (managing 14+ consultants in Germany, UK, Switzerland, Denmark, and Poland).

NNIT Facilitates Seamless Transition to New Electronic Data Capture Platform for Top 20 Biopharma Pioneer
News • Mar 18, 2024 15:00 GMT
NNIT completes successful collaboration with a Top 20 Biopharma pioneer in transitioning to a new Electronic Data Capture (EDC) platform for their clinical trials.
Recognizing the compelling benefits of the new EDC platform, the customer embarked on their transformational journey with meticulous attention to mitigating risks associated with moving active clinical studies data.
A seamless migration process was underpinned by NNIT's commitment to maintaining GxP data integrity and ensuring minimal disruption to ongoing clinical trials. Working closely with both internal C-level stakeholders, external partners, and health authorities, NNIT devised a rigorous validation and independent verification approach tailored to each active study migration.
100% Automated Verification of Study Configurations and Data Points
Utilizing NNIT’s proprietary Valiance TRUcompare™ software, the team executed 100% automated verification of all study configurations and each data point, enabling early identification of migration issues, and minimizing potential disruptions to study teams. This meticulous approach, combined with NNIT's extensive clinical data management expertise, ensured the delivery of trusted clinical trial data at scale.
"NNIT's clinical data management experience and data migration verification capabilities empowered our customer to transition smoothly to a single new EDC platform with confidence," says Greg Cathcart, Senior Vice President of the U.S. Region at NNIT, and he continues: "Our automated independent verification process played a pivotal role in safeguarding against unintended bias during the study data transfer process."
The adoption of NNIT’s faster, repeatable, predictable, and traceable migration verification process across the enterprise portfolio of active studies enabled the customer to circumvent the risks associated with parallel activity in twin EDC systems.

NNIT will move to new HQ during the summer of 2024
Pressrelease • Mar 01, 2024 07:30 GMT
NNIT’s headquarters will be relocating from Oestmarken 3A in Soeborg to Weidekampsgade 14 in downtown Copenhagen to become better connected to customers and partners and provide employees with a more inviting and open office environment.
The move to a new headquarters is a logical step on NNIT’s strategic journey to becoming the best possible digitalization partner for our customers – and at the same time becoming employer of choice.
Employees, customers, and partners can look forward to meeting in a physical environment that embodies who we are, and what we aspire to be.
CEO Pär Fors comments:
“Today, NNIT is a new company: As an IT consultancy, we have a stronger people core, and we are more international with our strong presences in Europe, Asia, and USA. With our new headquarters in downtown Copenhagen, we will be better connected, bringing us closer to our customers and partners, and allowing us to collaborate more closely in an inviting and open office environment. We are super excited and can’t wait to move into our new home.”

International HQ with city life at our doorstep
At our new location, we can also look forward to better connections to public and international transportation as well as proximity to great hotel accommodation, making it easier for our international colleagues to travel to our HQ.
Finally, with the city literally at our doorstep, and the lively Islands Brygge just a few minutes’ walk away, all visitors will be able to enjoy a wide variety of life options.

NNIT's Sustainability Report 2023: Contributing our expertise and capabilities towards creating a sustainable future
News • Feb 22, 2024 08:00 GMT
Every year, alongside our annual report, we issue a Sustainability Report (COP report), outlining the progress and direction of our sustainability activities.
Following the divestment of our now former infrastructure business, in terms of sustainability, 2023 was a year of certification, evaluation and a few new activities.
Sustainability Highlights for 2023
- In January, 2023, we achieved ISO 14001:2015 certification in Tianjin, China and Søborg, Denmark (Re-certified post-divestment in the summer of 2023).
- To align our sustainability profile closer to our New Beginning strategy, we focused on adopting a more streamlined and international approach. This included a widening of our Sustainability Committee to include representation from all regions as well as the appointment of regional Sustainability Ambassadors to help drive and create awareness about our sustainability work across our entire organization.
- The Sustainability Committee reviewed our SDG focus, once again to ensure alignment with our new strategy. It was decided to maintain our select focus on SDGs 4, 5, 9 and 12 combined with a plan to launch new initiatives to ensure sustainability engagement across all regions.
- Later in the year, a Diversity & Inclusion Community was established to help drive the D&I agenda forward in 2024 and beyond.
- Significant reductions in our CO2 emissions were achieved primarily as result of the divestment of data centers in Denmark. The work to achieve further reductions will continue in 2024 - in parallel with the work done to set Science Based Targets to be completed in August 2024.

Financial results for Q4 and FY 2023 released: Improved business and financial performance
Pressrelease • Feb 19, 2024 18:15 GMT
The Group improved business and financial performance throughout the year and delivered on the outlook, which was upgraded in August 2023.
Please refer to nnit.com - Investors for the full company announcement re the financial results for Q4/FY 2023.
CEO Pär Fors comments:
"2023 was a transformative year for NNIT as we relaunched our company as a pure-play IT consulting business highly specialized in global life sciences and the public sector in Denmark. We accelerated momentum throughout the year, confirming our confidence in the new strategic roadmap and enabling us to deliver on the promise of increased revenue and profitability. Looking into 2024, we see ample opportunities for growth within the life sciences space and a solid pipeline within the public sector in Denmark."
2023 key highlights
- Revenue grew by 15.2% (organic growth of 10.8%) to DKK 1,728 million following solid performance on existing engagements and onboarding of new customers across life sciences globally and the public and private sectors in Denmark.
- NNIT improved the operating result before special items significantly to DKK 116 million compared to a loss of DKK 7 million 2022 and lifted the operating profit margin before special items to 6.7% from -0.5% in 2022. Progress was driven by higher activity and capacity utilization combined with cost reductions.
- Special items amounted to DKK 69 million against DKK 278 million in 2022 and related mainly to earn-out payments.
2024 outlook
- NNIT expects to maintain the momentum from 2023 and continue to deliver organic revenue growth and profitability improvements in 2024 driven by good traction with customers and positive effects of efficiency measures.
- The Group expects to generate organic revenue growth of around 10% through expansion of engagements with existing customers and onboarding of new customers.
- The operating profit margin before special items is expected to increase to 8-9% based on improved capacity utilization, positive effects of increased use of nearshore and offshore capabilities, and by leveraging the regional structure and internal financial steering introduced in 2023.
Full company annopuncement is available at https://www.nnit.com/investors-media/investors/

Everest Group positions NNIT among the Leaders in the Life Sciences Digital Services Specialists PEAK Matrix® Assessment 2024
Pressrelease • Feb 05, 2024 07:30 GMT
Everest Group has just released its Life Sciences Digital Services Specialists PEAK Matrix® Assessment 2024 (part of the Life Sciences Information Technology research program), and we are proud to announce that NNIT features among the Leaders.
“NNIT emerged from life sciences, and all the virtues of one of the world's most regulated industries are deeply rooted in our DNA: We are experts, so it means the world to us to be recognized as leading life sciences digital services specialists. Operating in Asia, Europe, and the US, we are dedicated to life sciences, and every day we work to build our position as globally leading life sciences digital specialists – always seeking to empower those who change lives. This recognition is a testament to the massively talented team that is NNIT and the quality of the work we do as consultants driving positive digital innovation and change,” comments Senior Vice President Ricco Larsen, NNIT Life Sciences Global Solutions Development & Europe
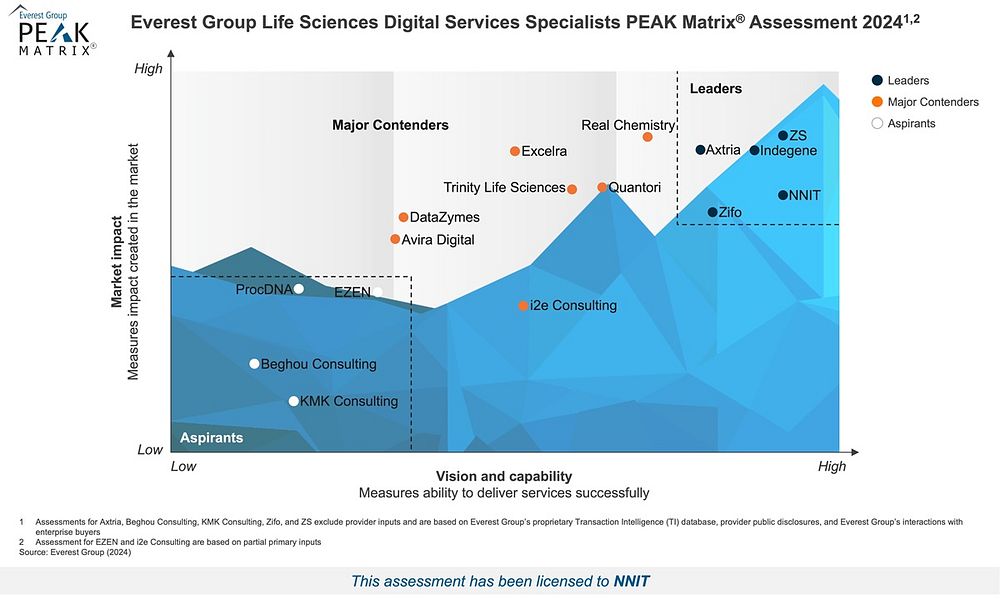
Strong Technology Credentials with Specialized Tools and Platforms
As part of the assessment, the Life Sciences Digital Services Specialists PEAK Matrix® Assessment 2024 highlights strengths and limitations for each specialist. As a Leader, NNIT receives the following appraisal:
“Buyers laud the domain expertise that NNIT offers through its digital solutions tailored to drug safety, clinical, quality, regulatory affairs as well as manufacturing and supply chain focus areas. Its arsenal of proprietary platforms and digital offerings has a panoramic coverage of the life sciences value chain from discovery to distribution”. Read more on the Life Sciences Digital Services Specialists PEAK Matrix® Assessment 2024.

New Partnership with Gimmal offers customers an Accelerated, GxP-Capable Information Governance Solution
News • Feb 01, 2024 08:30 GMT
Life science organizations face complex regulatory challenges, intensive audits, and robust internal process controls. With this new partnership, NNIT and Gimmal provide a well-established, compliant solution that customers operating within life sciences can rely on to manage the information lifecycle of GxP records.
The partnership combines the compliance advisory services of NNIT with the information lifecycle management solutions of Gimmal, allowing life sciences organizations to accelerate the implementation of a good practice (GxP) solutions to manage the quality and compliance of records and information with a defined validation process, especially for customers within the Microsoft ecosystem.
The new collaboration is off to a great start with its first successfully completed solution delivery for a leading global pharmaceutical company.
“NNIT has deep life sciences expertise, specifically within the compliance and validation arena, so we know how important it is to document and keep immaculate records. With Gimmal, we have found a very experienced and quality-oriented partner that offers a top-of-the-line information governance software platform. Together, we can offer a unique and accelerated GxP-capable governance and compliance solution,” Ricco Larsen, Senior Vice President, NNIT Life Sciences Global Solutions Development & Europe, explains.
“Leveraging Gimmal's renowned information governance software platform, this collaboration with NNIT ensures the traceability and accountability in critical GxP processes, including leveraging our deep knowledge and experience meeting FDA 21 CFR Part 11 regulatory requirements in the US, for both paper and electronic records,” Craig Carpenter, CEO, Gimmal adds.
Join NNIT and Gimmal for an Educational Webinar
NNIT and Gimmal will be co-hosting an educational webinar in March 2024, more details about this will be published shortly. Follow and keep an eye on our LinkedIn page to get updates on this.
***
About Gimmal
Gimmal is Information Governance, simplified. As the market’s only end-to-end information governance software platform, Gimmal helps enterprises and government agencies tackle their most complex,mission-critical information governance challenges at scale across the full information lifecycle. From information classification to migration, and data discovery to remediation, learn more about Gimmal’s comprehensive information governance solutions at www.gimmal.com

Catalyst for Continuous Growth in the US
Pressrelease • Jan 30, 2024 12:00 GMT
As NNIT expands its presence in the United States, particularly with the anticipated integration of Excellis Health Solutions (Excellis), Greg Cathcart, Head of Excellis, has assumed the lead role of Senior Vice President to accelerate growth and advance the market position in the US.
In the past five years, NNIT has substantially expanded its US presence through organic growth and strategic mergers and acquisitions (M&A). Excellis, acquired in 2020 to enhance NNIT's strategic consulting services portfolio, is now being integrated to expedite NNIT's growth journey in the US market.
End-to-end life sciences consulting services for every step of the value chain
The new NNIT US organization will employ over 200 professionals and a range of subject matter experts in almost all areas of life sciences operations while offering solutions spanning the life sciences value chain, including Regulatory, R&D, Quality & Compliance, Manufacturing & Supply Chain and Data & Digital, with plans to expand the solutions portfolio further.
“From the beginning, we saw great potential for synergies. The combination of NNIT’s end-to-end life sciences value chain consultancy services with Excellis’ specific focus on serialization and track & trace globally is second-to-none. It is time to take our union to the next level, becoming one company and taking full advantage of those synergies to unlock further growth. I am excited and proud to be heading up this new era for NNIT and our many talented people in the US,” comments Greg Cathcart, in his new role as SVP, Head of NNIT US.
Supporting future growth
As the integration of Excellis gets underway, and complimentary teams, experts and professionals are coming together to create a unified culture, 2024 is poised to be a significant year for NNIT US. At the same time, to support plans to grow both top and bottom lines, NNIT US will onboard up to 25 new professionals to bolster an already dynamic team.
“We operate in a very meaningful space empowering those who change lives, and we’re growing beyond our long-serving and loyal teams, so it’s an exciting time to join NNIT US. We’re constantly enhancing our diverse workplace where we use our domain expertise to create results for our customers. We’re looking for individuals with an appetite for new adventures and the opportunity to liberate their talent and make a mark,” Greg Cathcart says.
Organic growth and M&A
In accordance with the NNIT Group strategy, a strong focus is on continued organic and acquired growth in the US, specifically the ambition is to grow the solutions portfolio and add further areas of strategic consulting expertise in support of the life sciences value chain via strategic M&A.
“We’re absolutely expecting to reap the benefits from the combined NNIT and Excellis teams and expand our offering to the life sciences industry. We’re going to thoroughly analyze our current solution portfolio with a view to making our existing services more robust. There’s a lot of potential for cross-pollination here – and we’re all very excited about our prospects,” Greg Cathcart finishes.
About Greg Cathcart
A significant portion of Greg Cathcart’s early career was spent at Johnson & Johnson in manufacturing and supply chain roles. In 1991, he made a transition to a tech company specializing in ERP solutions (QAD Inc) for pharmaceutical and medical device companies. In 2002, Greg joined SAP where he led the global life science practice for five years. His inherent entrepreneurial spirit eventually led him to establish Excellis in 2008. He sold Excellis to NNIT in 2020 – and having over-performed on the earnout agreement, he now continues as Senior Vice President, Head of NNIT US.

NNIT is Denmark's first Certified Training Partner for OutSystems Low-code
News • Jan 08, 2024 09:27 GMT
In January 2024, NNIT became the first certified training partner for the OutSystems low-code platform in Denmark. This important achievement is in line with our strategic commitment to great educational experiences where we want to fully support the technological proficiency in the Danish region.
“With numerous successful training sessions already under our belt, NNIT is now positioned to elevate these offerings to new heights. By harnessing officially approved materials directly from our partner OutSystems, we ensure the highest standards of quality in both execution and content. Our approach guarantees that each training session is not just informative but also transformative, aligning perfectly with the specific needs and goals of our customers,” says Mette Thorning, Solutions Architect, Custom Application Development in NNIT.
NNIT has more than 25 developers and consultants already working on large low-code projects with several customers in both the public and enterprise sectors in Denmark. The agile set-up and the flexibility low-code offers for custom application development projects has proved to be the right match for many customers wanting to focus on technological empowerment and building solutions for the future.
Low-code training for education and certification
With our instructor-led training, delivered by certified trainers, NNIT now offers various forms of training in low-code in a both flexible and effective set-up. Whether in a classroom setting, remotely, or through a hybrid model, we adapt to the most convenient format for each customer, delivering in-depth knowledge leading to certification and a comprehensive skill set that can tackle the challenges of tomorrow.
In addition to an official low-code bootcamp, NNIT also offers extended courses on advanced topics in OutSystems aimed at companies looking to expand their capabilities on an existing OutSystems platform or for those embarking on a migration journey to OutSystems.
Understanding the diverse needs of teams, we offer low-code training in both Danish and English fitting both purely educational needs as well as the official certification process based on OutSystems.

NNIT to offer end-to-end ESG Advisory Services with partners IBM and Transition
News • Dec 19, 2023 07:30 GMT
Having already partnered with IBM on the implementation of a finance-grade ESG system, NNIT now onboards sustainability experts Transition to provide end-to-end ESG advisory services.
With the aim of providing customers with the complete ESG advisory service package – from ESG analysis and strategy to data collection and management and to IT system implementation and communication – NNIT and Transition have entered into a new partnership:
“NNIT are experts in IT development and implementation within regulatory domains, and together with IBM’s Envizi software, we believe we have the best finance-grade ESG system offering in the market. With Transition joining us, we will be able to attach some of the foremost ESG experts to our advisory services and process, making sure that we provide deep domain expertise every step of the way towards compliant reporting and communication on progress when it comes to companies’ sustainability agenda,” says Head of NNIT ESG Advisory Services Lars Dinesen.
“When advising companies on the new EU regulation on ESG reporting, we see that it is imperative that companies structure and digitalize their ESG efforts in one single tool. With NNIT as the implementation partner, we will be able to provide that end-to-end solution, assisting companies in navigating new regulation and improve their ESG performance. We are very excited by this new partnership and the positive impact it can bring,” comments Head of ESG at Transition Mathias Selchau Majlund.
Join us in February for our ESG Seminars in Soeborg or Aarhus
On February 7 and 8, 2024, NNIT will be hosting two ESG seminars with partners IBM and Transition in Soeborg and Aarhus, respectively. The morning seminars have a packed agenda centering on how you take ownership of your ESG reporting and drive impact and change.
About Transition
Transition are experts in sustainability and offer a wide variety of sustainability services. Together with NNIT and independently, they assist companies and organizations in navigating the many ESG reporting frameworks and protocols. Using their deep domain insight into different markets, EU regulation and stakeholders, they support customers from A to Z – from materiality assessment, gap analysis and data collection to reporting and communication.

Mohammad Daniali takes silver medal in the IT consultant category at the Version2 IT Talent 2023 Awards
News • Dec 08, 2023 11:56 GMT
Each year, Danish Tech publication Version2 calls for nominations of IT talents. The competition is subject to comprehensive nominations, screening and the scrutiny of a professional jury. The nomination categories included:
- IT Developer
- IT Project Manager
- IT Consultant
NNIT's Mohammad Daniali was nominated in the category of IT Consultant, and he came in 2nd in the top 10 list of IT Talents in Denmark.
A big surprise to be nominated
Mohammad Daliali reports that he was really surprised when he was advised he had made the shortlist. "I actually thought it might be a phishing email, but when I realized it was legitimate, I was just really happy to be acknowledged, and then I had to keep it a secret for a month."
He continues:
"Yesterday [at the award ceremony], I felt priviledged to be among the other winners, but I must acknowledge my team in this, because the award represents a shared success built upon dedication, innovation, and the collaboration of my exceptional team. In my acceptance speech, I was also quick to recommend IT consulting within life sciences, because technology plays a pivotal role in shaping the future of healthcare, and I've been fortunate to contribute to this field, leveraging technology to optimize processes, enhance research, and ultimately improve patient care."
Technical prowess, customer-centric and commitment to results
Mohammad Daniali's boss put the nomination and win into choice words on LinkedIn:
"Internally, Mohammad Daniali is celebrated as an outstanding IT consultant, Mohammad boasts exceptional technical prowess, a client-centric approach, and an unwavering commitment to delivering results. Mohammad's exceptional achievements in 2023 include leading the development side of the implementation of POMSnet, an agile web-based manufacturing software (MES), for a major pharmaceutical client in Europe. His dedicated efforts will lead to a streamlined, digital, and modern efficient manufacturing process." - Daniel Fitussy, Director, NNIT Manufacturing and Supply Chain Consulting.
Congratulations Mohammad!

Casper Cambell Kjøller rejoins NNIT as new Vice President, Head of Sales for Region Denmark
News • Nov 30, 2023 09:00 GMT
NNIT’s Sales unit in Region Denmark covering the Private and Public Sector is now headed by Casper Cambell Kjøller who is a well-known face as he has previously been with NNIT for 7 years, working as a Sales Executive within the public sector in Denmark.
Casper Cambell Kjøller, Vice President, Head of Sales in Region Denmark, has more than 20 years of experience working with the public sector in Denmark and will be responsible for driving the new strategic direction focusing on growing NNIT’s business in the Public sector while still keeping the focus and momentum in the Private sector.
Casper C. Kjøller comes to NNIT from a position as Sales Director for the Public sector at Accenture and brings broad Public sector sales experience from NNIT, KMD & Terma.
"I wanted to rejoin NNIT because of the new strategy focusing on being an international IT consultancy where development and innovation go hand in hand, and where we can more seamlessly drive the digital transformation journey of our customers. And getting the chance to be part of a company where I know from experience that the values really matter and bring out the best in our people and culture was also a key reason to come back," comments Casper C. Kjøller.
Creating real value with IT developed for people by people
As part of the new strategy for NNIT, an important strategic goal for Region Denmark is to grow the business in the Public sector where Casper, in his own words, sees a great match for the customers and for NNIT.
"While we of course want to keep building our business in the Private sector, we see a strategic potential in growing in the public sector, which makes sense since we have all the right knowledge and experience to help the public customers with their digital transformation and custom applications. Over the years, we have gained significant experience from various public customers with critical Danish IT infrastructure and really know how to develop solutions "for people by people," as we say. Creating real value by designing and developing IT for the people who depend on using the solutions daily," says Casper C. Kjøller.
And the future looks promising with everything from AI, Cloud, Cybersecurity, MS & SAP services to Custom Application Development or Low Code as part of the many trends forming digital transformation and innovation these years. Subjects that Casper and his team look forward to discussing at the two big events next year – the conference OffDig in March and Folkemødet on Bornholm in June 2024.

Change in Mangement
News • Nov 27, 2023 17:03 GMT
Having relaunched NNIT with a new strategy, and as part of that completed the transformative divestment of the infrastructure operations, we are now introducing a change to Group Management as Executive Vice President Pernille Fabricius has decided to leave NNIT end of November 2023 to pursue opportunities outside NNIT.
Pernille Fabricius joined NNIT in 2020 as CFO, and later she transitioned into a role as EVP, Head of Strategy, Transformation and M&A.
Pär Fors comments:
“As part of the NNIT Management Team, Pernille Fabricius has had an intense focus on transforming NNIT and transitioning the business to what we are today: A focused IT consultancy specialized in life sciences internationally and the public sector in Denmark. I wish to thank Pernille for her contributions in supporting the transaction and leading NNIT through times of great change. I wish her the best in her future endeavors.”
Balancing organic growth with M&A
As announced in our ‘New Beginning’ Strategy and at our Capital Markets Day, we are focused on both organic growth and M&A activity, and going forward the M&A responsibility will be anchored in our CFO office headed by Carsten Ringius.
Having successfully finalized the divestment of the infrastructure operations, NNIT is secure in its strategy and will continue to focus on becoming the best possible digitalization partner in industries where quality of life is at play – winning market share in life sciences internationally and the public sector in Denmark and securing profitable growth.

Financial results for Q3 and 9M 2023 released: Solid revenue growth of 19%
Pressrelease • Nov 09, 2023 07:57 GMT
The positive trajectory from the first half of 2023 continued through the third quarter. The improvement of performance is in line with the upgraded guidance provided in August and emphasizes the positive direction set with the new strategy.
Please refer to nnit.com - Investors for the full company announcement re the financial results for Q3/9M 2023.
CEO Pär Fors comments:
“Q3 is the first full quarter after the launch of the new strategy, and we are really pleased to see a continued positive development in our business performance, revenue and profitability. This further supports that we are on the right track with our new strategic direction, and we remain fully focused on delivering in accordance with our guidance and longer-term aspirations.”
- Solid revenue growth of 19% to DKK 453m compared to Q3 2022 driven by more business with existing customers and new logos.
- The group operating profit before special items increased to DKK 26m, equal to a group operating profit margin of 5.8%. The margin expansion of 5%-points compared with the same quarter last year is due to improved business performance leveraging a lower cost base.
- The 2023 financial outlook is unchanged: Revenue growth of around 15% and group operating profit margin excl. special items of around 6%.
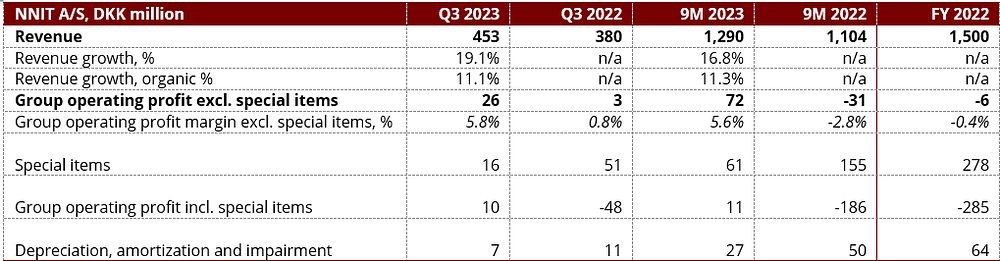
Business Review: Solid revenue growth and improving profitability
In Q3 2023, NNIT delivered solid revenue growth of 19% across the Life Science and Public-Enterprise spaces, compared with the same quarter last year, driven by growing existing engagements and bringing in new logos. Furthermore, NNIT continues to see solid growth coming from the group companies Excellis Health Solutions, SL Controls and SCALES. The revenue generated towards Aeven after the split and sale of the infrastructure business end of April is considered inorganic revenue in the reported trading statement.
Group operating profit excluding special items was DKK 26m compared with DKK 3m last year. The group operating profit margin excluding special items ended at 5.8% versus 0.8% in Q3 2022. Margin improvement of 5%-points compared with last year was primarily due to lowering of regional and group overhead cost.

New Corporate Sustainability Reporting Directive calls for a new approach
News • Sep 22, 2023 08:30 GMT
For the past many years, corporate sustainability work and reporting have been more or less ungoverned and unaudited (under the Non-Financial Reporting Directive). Soon, with EU’s Corporate Sustainability Reporting Directive (CSRD) coming into effect in January 2024, the scope of requirements will be significantly extended, impacting up to 50,000 European companies.
With the CSRD, the focus will shift from framework commitment and reporting to data and metrics reporting as well as a requirement for third-party assurance and external auditing. This will greatly increase the need for data measurement and management – and within a relatively short period of time too.
Companies are looking at the expensive options of staffing up and/or automating data management and reporting, with neither option necessarily providing a means of recuperating from the added costs – much less keeping a firm focus on driving sustainability progress.
Is the outlook really that grim?
We sat down with Lars Dinesen, Head of NNIT’s ESG Advisory Services for some input on how companies might turn the prospect of increased regulation and administration into a competitive advantage.
Leapfrogging beyond compliance to a competitive advantage
“What if we looked beyond the compliance aspect of our sustainability work? What if we invested in a future where our sustainability efforts generate value for investors, customers, employees and society at large – and we had the data at hand to prove it on a continuous basis,” Lars Dinesen rhetorically asks and kicks off the conversation.
He calls for a radically different approach to sustainability work and reporting, specifically when it comes to reporting practices with the goal of change rather than just compliance:
“Why is it that we’re satisfied with reporting on our progress only once a year? Why not measure and demonstrate our impact – or when it comes to the environment reduced impact – on an ongoing basis? With constant measurement and reporting, we can both communicate and act more proactively to secure a better future,” he asserts.
He is talking about NNIT’s new partnership with IBM on the Envizi ESG suite; a dedicated, automated and auditable ESG reporting system offering financial grade data management and reporting in addition to dynamic dashboards to drive ESG change and excellence.
New progressive ESG tool on the block
As a leading digitalization partner, NNIT looks for opportunities to transform customers’ business with the right IT solutions to help them continually release their potential. This is especially important when impacted by new regulation requiring 100% compliance, but also in mitigating the risk of stalling all progress because of increased administration.
Lars Dinesen and team believe they have found the right solution to the upcoming ESG reporting challenge – a solution that also looks to our sustainable future:
“With the Envizi ESG suite, companies don’t just get an ESG reporting system, they get a sustainability system that can drive the business forward by bringing their efforts and results to light, enabling key stakeholders such as investors, customers and employees to actively engage with the company,” Lars Dinesen states and adds:
“We need to move away from the manual processes and invest in a better future – both from a business and society point of view. The CSRD is coming into effect, but so are climate changes and new standards for corporate social responsibility, and we need to get in front of them rather than lagging behind and trying to catch up.”
Invest now, save in the long run
As with ERP systems, when they were introduced to the market, a progressive and fully compliant ESG system comes with an initial investment.
“On the bright side, once implemented, the Envizi ESG suite will automate most of the previously hand-held data collection and management processes, and it will ensure 100% compliance with ESG regulation as well as provide accurate data to allow you to communicate sustainability stories without any risk of ‘washing,’ because it’s simply stating the validated facts,” says Lars Dinesen.
He continues: “The Envizi ESG suite was built by ESG specialists, it incorporates CSRD and ESRS and comes with strong data management capabilities, so you will be able to drastically reduce the need for consultants. Once configured, the system does most of the ESG consulting for you. If you want to move beyond the compliance aspect and save on costs, frankly, it’s the only way to go.”
Get strategic with increased transparency
According to Lars Dinesen, another added bonus when you drive your sustainability activities similar to the way you drive your financial top and bottom line is that it will ensure the engagement of all the right stakeholders inside and outside the business.
“With increased transparency and data validation, and your targets plotted into the system, you will know exactly where you’re at as well as where you need to go. And you will be able to communicate this progress – or lack thereof – to the right stakeholders on an ongoing basis, thereby engaging them on the path to change. I think we all want to get engaged, but we need more timely information in order to know what to do and when to act,” Lars Dinesen says and ends:
"Of course, in order to get started, the ambition must go beyond compliance, and it can be difficult to convince our stakeholders before we are able to provide the transparency and added value that comes with the system. But we will keep pushing this, because we know it is the only way to turn a compliance exercise into a competitive advantage.”

Financial results for Q2 and H1 2023 just released: Group revenue increased by 15%
Pressrelease • Aug 31, 2023 11:34 GMT
NNIT completed the transformative divestment of its infrastructure operations to become a highly specialized IT services provider and generated growth and improved earnings entailing an upgrade of the 2023 outlook on August 26.
Please refer to https://investor.nnit.comfor the full company announcement re the financial results for Q2/H1 2022.
CEO Pär Fors comments:
“Having launched our new strategy to become a highly specialized IT services provider, after the successful divestment of our infrastructure business, we saw further positive results in Q2, supporting our choice of direction for the future. NNIT delivered strong performance and organic growth based on solid business performance, improved utilization, and adjusted costs, which led to an upgrade of our 2023 outlook on August 26.”
- Group revenue increased by 15% to DKK 424 million in Q2 and by 16% to DKK 837 million for 6M 2023.
- The operating profit before special items increased to DKK 25 million for Q2 from DKK -18 million in Q2 2022 and increased to DKK 45 million for 6M 2023 from DKK -34 million for 6M 2022. The increase is driven by improved business performance, better utilization and lower costs. As a result, the operating profit margin before special items increased to 5.9% for Q2 2023 from -4.8% in Q2 2022 and to 5.4% for 6M 2023 from -4.7% for 6M 2022.
- Special items amounted to DKK 30 million for Q2 and DKK 44 million for 6M 2023 mainly related to the change in accounting treatment of earn-outs following a decision by the Danish Business Authority. The decision has been appealed by NNIT.
Business highlights
- NNIT continued to organically grow its current business by expanding its engagement with several clients and growing through group companies such as SCALES and Excellis Health Solutions.
- To support strategy execution, focusing on the life science industry internationally and on the public sector in Denmark, NNIT introduced a new regional organizational structure aiming to increase customer proximity and enhance global coordination across regions. The Group’s financial reporting format was subsequently changed to reflect the new organizational structure, cf. company announcement no. 20/2023 released on August 30, 2023.
Outlook
- NNIT upgraded its full-year outlook for 2023 on August 26, cf. company announcement no. 19/2023. Revenue growth is expected to be around 15% (previously around 10%) with an operating profit margin before special items of around 6% (previously around 5%).
- Special items are still expected up to DKK 70 million in 2023 relating to earn out payments connected to acquisitions and restructuring costs to right-size the organization after completion of the divestment of the infrastructure operations.

Christian Staalby joins NNIT as new Head of SAP
News • Jun 26, 2023 08:30 GMT
NNIT’s SAP unit is now headed by Christian Staalby who has spent most of his career either working with or being employed by SAP.
Christian Staalby, Vice President, Head of SAP, will be responsible for setting the new strategic direction of SAP within NNIT’s new Region Denmark focused on the public, enterprise and life sciences sectors.
Christian Staalby comes to NNIT from a position as Client Partner at Cognizant. Before that, he held executive positions at Charlie Tango and Kony following an 11-year tenure at SAP.
“SAP is a part of my DNA, it’s where I started my career; it was the center of my work throughout my 11 years working for SAP, and it has stayed with me in some form or other ever since. NNIT and SAP go back a long time, and I look forward to setting the new direction for the continued partnership with SAP. We have a solid SAP business with highly skilled functional experts, experienced integration specialists and great customer engagements across different industries, and we will leverage this when we build the new NNIT SAP. Going forward, we will be much more focused on servicing both the public, enterprise and life sciences markets doing business transformation projects with SAP – and we are both ambitious and innovative in our approach," comments Christian Staalby.
“Having divested our former SAP Basis business with the Aeven deal, we are now looking to reinvigorate our SAP offering to the Danish market. In Christian Staalby, we have secured one of the strongest SAP leaders to drive our growth agenda within this space. He is the perfect candidate to release our potential here," says Senior VP, Kasper S. Andersen.

Lars Verning to head up NNIT Cloud
News • Jun 20, 2023 08:30 GMT
In June, we have added Lars Verning to our Microsoft Dynamics 365 team. He will assume the position of Associate Vice President and head up the NNIT Cloud solution area.
Lars Verning comes to NNIT from Microsoft where he held the position of Enterprise Channel Manager. He also has extensive experience from IBM and SAS Institute where he held positions as Country Manager and Software Channel and Sales Director.
In his own words, Lars Verning specializes in “advising customers on how to unlock the value of the Microsoft Cloud. From Cloud foundation to the use of various technologies like Data, AI and IoT.”
”We are delighted to add Lars Verning to our team. His strategic vision within Cloud, Security and AI, and his experience in executing business plans, will be central to launching the new strategic direction of NNIT Cloud – and, most importantly, in accelerating our customers’ transformation journey,” says Gaurav Anand, Vice President, NNIT Microsoft Advisory & Technology.
“Joining the new NNIT, after the Aeven divestment, and adding the Cloud element to the NNIT Microsoft Powerhouse is an exciting task. Taking the last three years of dialogues with Microsoft customers about needs and challenges and converting them into Microsoft Cloud Services will be my main objective. NNIT certainly has the capabilities to be a Top 3 Microsoft Cloud Partner in all known areas – we just need to assemble the elements in new ways and possibly add a few new services to our existing and extensive catalogue, says Lars Verning, Vice President, NNIT Cloud.

Ebbe B. Petersen is NNIT’s new Chief Information Security Officer
Pressrelease • Jun 01, 2023 10:00 GMT
Following the recent emergence of NNIT as an international IT consulting business focusing on life sciences internationally and the public and enterprise sectors in Denmark, NNIT has appointed a new Chief Information Security Officer (CISO).
The appointment of Ebbe B. Petersen is effective immediately; and in this new role, he will report directly to CEO Pär Fors. At the same time, Ebbe B. Petersen will continue as Head of NNIT’s Cybersecurity and Compliance unit.
CEO Pär Fors comments:
“Cybersecurity has never been more top of mind for all of us. Companies and citizens are exposed to cyber threats every single day, and unfortunately attackers continue to be successful in penetrating even the most advanced defenses. As an IT consulting company, advising on cybersecurity, NNIT needs to be the best example. With the appointment of Ebbe B. Petersen as CISO, we will be practicing exactly what we preach to our customers on cybersecurity.”
CISO Ebbe B. Petersen adds:
“During times of crisis – with market turbulence, war and general political instability – cybercriminals are more active, because the chaos and uncertainty makes it easier to launch attacks. In addition, the ever-increasing evolution of technology and commercialization and organization of cybercrime make security a top priority. As society and businesses become more digitalized, we all face new security challenges, which we must address in order to continue to be safe and successful. This is not least true for the safeguarding of our critical infrastructure. In my new role, I will focus on continued optimization of the strong, proactive cybersecurity setup NNIT has developed over the years. In order to effectively minimize risks for our customers, partners and NNIT, it is crucial that we are always prepared to prevent, mitigate and recover as new attacks surface.”

New General Managers in the Philippines and Czech Republic
News • May 16, 2023 07:30 GMT
Following the divestment of our former infrastructure operations, two new general managers have been appointed to head up our service delivery centers in the Philippines and Czech Republic.
Pio Consuelo has been appointed General Manager of NNIT Philippines, and Danijel Marjanovic has been appointed General Manager of NNIT Czech Republic.

Pio Consuelso and Danijel Marjanovic have both been promoted from their former NNIT roles of Director for the Global Delivery Transition Project (SAP area) and Head of the Life Sciences Europe engagement team, respectively.
Going forward, they will drive the new organizations in close collaboration with NNIT’s regions and ensure their teams get off to a good start as part of the new NNIT – an international IT consulting business focused on life sciences internationally and the public and enterprise sectors in Denmark.
“We are very happy to promote Pio and Danijel who have demonstrated strong leadership and talent in their previous roles – and to have such capable people heading up our global delivery centers. They will play a key role in ensuring that we continue to deliver to the high standards NNIT is known for whilst we accelerate the growth of these teams to reinforce our competitive edge in our chosen markets,” says Senior VP, Head of Region Denmark, Kasper S. Andersen.

Introducing a new NNIT management team
Pressrelease • May 11, 2023 08:30 GMT
NNIT presents a new management team – following the divestment of its infrastructure operations and launch of a revised strategy to support the new beginning as an international IT consultancy business.
The new management team consists of ten members. In addition to CEO Pär Fors, CFO Carsten Ringius and Executive VP Pernille Fabricius, four regional heads and two global sales and solutions heads have been added to successfully lead NNIT as an international IT consultancy business focused on life sciences internationally and the public and enterprise sectors in Denmark.
The new NNIT management team
- CEO Pär Fors
- CFO Carsten Ringius
- Executive VP, Head of M&A Pernille Fabricius
- Senior VP, Head of HR, Signe Nelsson (Starts June 1)
- Senior VP, Head of Region Asia, Jason Xing
- Senior VP, Head of Region Europe, Ricco Larsen
- Senior VP, Head of Region Denmark, Kasper Søndergaard Andersen
- Senior VP, Head of Region USA, Mark Ohrvall
- Senior VP, Head of Global Sales, Flemming Kjoller
- Senior VP, Head of Global Solution Development, Ricco Larsen (Acting)
Global organization, regional empowerment
By creating an international top management, introducing a new locally empowered regional structure coupled with global sales and solution development organizations, we will increase proximity to our customers. That is, we will be locally anchored, close to our customers and their needs, while drawing on our global sales and solutions capabilities to ensure continued high quality and scalability in our deliveries.
“We are proud of this new, strong management team that represents our international presence and our desire to always be close to and in dialogue with our customers. We are also very happy that it is a more diverse management, more representative of our workplace and employees across Asia, Europe and in the US. We believe it is a key step in becoming the best possible digitalization partner for our customers and workplace for our people,“ says CEO Pär Fors.

Trading Statement for the first three months of 2023 released
Pressrelease • May 04, 2023 11:34 GMT
NNIT completed the carve-out and divestment of its infrastructure business – the single most transformative event in company history – on April 28. The Group delivered solid business performance, generating revenue growth of 16% (11% organic) to DKK 413 million in Q1 2023 and improved the operating profit margin significantly to 4.9% on the back of higher revenue and utilization. NNIT maintains the financial outlook for growth and operating profit before special items for 2023.
Pär Fors, CEO of NNIT, comments:
"We are pleased to report solid business performance and stronger financials in the first quarter of 2023 as Life Sciences Solutions and Cloud and Digital Solutions lifted the activity level and improved utilization. We won several new contracts and renewals and remain on track to deliver our 2023 guidance. Equally important, we are thrilled that the divestment of our infrastructure business was completed on April 28, 2023, paving the road for the development of NNIT as a highly specialized IT services provider with two strongly positioned business units”.
Highlights Q1 2023
Financial highlights
Revenue increased YoY by 16% (11% organic) to DKK 413 million in Q1 2023, driven by strong contributions from both business units with particularly solid growth in the two areas Production IT and Custom Application Development.
The operating result before special items increased to DKK 20 million from DKK -16 million positively impacted by growth and improved utilization. The operating profit margin increased to 4.9% compared to -4.5% in Q1 2022.
Special items amounted to DKK 14 million, mainly attributable to earn out payments related to acquisitions following a decision by the Danish Business Authority.
Business highlights
NNIT successfully completed the divestment of the Group's infrastructure business on April 28, 2023, at a purchase price of DKK 1,350 million (Enterprise Value on a debt free basis) after EBIDTA adjustments. In line with expectations, the estimated cash impact of the transaction, adjusted for net debt and working capital, carve-out and separation costs as well as estimated tax, amounts to DKK 1,288 million including a vendor note of DKK 200 million, which will be repaid no later than six years after closing.
Significant new contracts and renewals secured across Life Sciences Solutions and Cloud & Digital Solutions, including two public contracts and several long-term engagements with international life science customers impacting 2023-2025.
Outlook
NNIT maintains the outlook for growth and operating profit before special items for 2023 with revenue growth of around 10% and operating profit margin before special items expected to increase to around 5% through higher revenue and improved utilization.
NNIT still expects costs of up to DKK 180 million in 2023 relating to carve-out and separation activities as well as earn out payments connected to acquisitions (following a decision by the Business Authority) and restructuring costs to right-size the organization after completion of the strategic divestment of the infrastructure operations. Following closing, NNIT expects to book up to DKK 70 million of these costs as special items with the remaining amount being re-classified as part of discontinued activities.

NNIT completes transformative divestment of infrastructure operations to become a highly specialized IT services provider
Pressrelease • Apr 28, 2023 11:41 GMT
Today, NNIT has completed the transformative divestment of the Group’s infrastructure operations to funds advised by Agilitas Private Equity LLP.
The overall scope of the divestment of the infrastructure operations is unchanged except that the Group’s Chinese activities, including shares in its Chinese subsidiary, will remain with NNIT.
Following closing of the transaction, the refocused NNIT will encompass approximately 1,700 skilled employees in the two business areas Life Sciences Solutions and Cloud & Digital Solutions, delivering high-quality solutions and services in attractive markets with strong growth prospects – and operating in Denmark, the Czech Republic, Germany, Switzerland, the UK and Ireland, USA, the Philippines, China, Singapore and Poland. The transformative divestment serves as a pathway to become a highly specialized IT services provider.
The purchase price (Enterprise Value on a debt free basis) for the divested operations is DKK 1,350 million after EBITDA adjustments at closing of the transaction.
Pär Fors, CEO of NNIT, comments:
”We are thrilled to successfully complete the divestment of the infrastructure operations after months of dedicated work, enabling us to pursue exciting new opportunities through industry mastery and delivery of superior quality. This transformative transaction is a major step in our efforts to reshape NNIT and emerge as a highly specialized IT services provider exclusively focused on the Life Sciences and Cloud & Digital Solutions business units in globally attractive markets with a high demand for our unique competences and proven ability to navigate complexity. We look forward to embarking on this new beginning for NNIT and developing our business to drive growth, deliver operational excellence and improve profitability.”
